How Can I Tell if My Babies Eyes Are Dialited
Abstract
Sensitive responding to heart cues plays a key role during man social interactions. Observed changes in pupillary size provide a range of socially-relevant information including cues regarding a person's emotional and arousal states. Recently, infants have been found to mimic observed pupillary changes in others, instantiating a foundational mechanism for centre-based social communication. Among adults, perception of pupillary changes is affected by race. Here, nosotros examined whether and how race impacts the neural processing of others' pupillary changes in early ontogeny. We measured ix-month-old infants' brain responses to dilating and constricting pupils in the context of viewing own-race and other-race eyes using functional near-infrared spectroscopy (fNIRS). Our results show that but when responding to ain-race eyes, infants' brains distinguished betwixt changes in pupillary size. Specifically, infants showed enhanced responses in the right superior temporal cortex when observing own-race pupil dilation. Moreover, when processing other-race pupillary changes, infants recruited the dorsolateral prefrontal cortex, a encephalon region linked to cognitive control functions. These findings suggest that, early in development, the fundamental process of responding to pupillary changes is impacted by race and interracial interactions may afford greater cognitive control or attempt. This critically informs our agreement of the early origins of responding to pupillary signals in others and further highlights the impact of race on the processing of social signals.
Introduction
The ability to detect and respond to data from the eyes is an early developing capacity that is considered a foundational feature of human social noesis in infancyone,ii. During face-to-face social interactions, information regarding a person's attentional, emotional, and mental state can be gleaned from the middle region and from the pupillary land3,iv,5. By adulthood, a preference develops for individuals with larger pupils and pupil dilation is recognized as a point for positive touch on4,half-dozen. Though this preference has been shown to emerge in early adulthood, much less is known about how infants perceive and respond to pupillary cues. Contempo piece of work shows that early in ontogenesis humans already differentially respond to changes in others' pupil size (diameter). Specifically, infants as immature as 4 months of age brandish greater educatee dilation when viewing photographs of dilated eyes, suggesting pupil dilation mimicry7. Similarly, infants six to nine months of age show greater pupil dilation when viewing larger compared to smaller schematic optics7,viii. Considering that changes in pupil size are closely tied to changes in physiological arousal controlled by the autonomic nervous system, it is possible that the coordination of arousal between social partners facilitates student mimicry or alternatively that pupil mimicry facilitates the unconscious coordination of arousal between social partners6,7,viii,9,ten. Regardless of the verbal directionality of how pupil mimicry and the coordination of arousal between social partners are linked, responding to others' pupillary changes has been argued to play an important role in guiding and impacting interpersonal contactxi. More specifically, from a developmental perspective, although the same studies with infants attest that pupil dilation mimicry exists early on in ontogeny, many question remain regarding the nature of infants' sensitivity to others' pupillary changes.
Importantly, enquiry with adults shows that pupil mimicry between social partners depends on race. Specifically, in Kret, Fischer, and De Dreu's written report, adults displayed greater educatee dilation mimicry for own-race members than for other-race members. Moreover, this study found that adults trusted others with dilating pupils more, but this effect on trust was only seen when responding to ain-race partners' eyesxi. These findings emphasize that the relation between pupillary cues and overt, social behavior are impacted by race in adults. Moreover, these results are interpreted as adults beingness more than sensitive to social signals in the context of their own-race due to biases that have adult over time. Yet, information technology is unknown whether just lack of familiarity, and not the protracted evolution of racial biases, influence the detection of pupillary cues. The 2d goal of the current study, therefore, was to examine the impact of race on infants' responses to observed pupillary changes. Already by iii months of historic period infants have been shown to visually prefer own-race over other-race faces12. By around nine months of age, perceptual narrowing to familiar race faces occurs in infants, resulting in impeded identity and emotion recognition from other-race faces13,xiv. Furthermore, between four and 9 months of age, infants' fixations on internal facial features decrease only in response to other-race faces just non to ain-race faces15. Based on previous work showing that by around 9 months of age infants' processing of social information (identifying individuals and emotion discrimination) is intact for ain-race faces but impeded for other-race faces13,xiv, we decided to study infants at nine months of historic period. Given the role of attentional processes and experience, nosotros likewise assessed how attending to stimuli and other-race exposure affects the response to own- and other-race pupillary cues.
It is important to note that infants at this age practise prove a visual preference for own-race faces but practice not yet testify racial biases in their overt social behavior. More specifically, prior work has shown that ten-month-old infants were equally probable to take a toy from an own-race when compared to an other-race membersixteen. In this study, 24-month-old infants were besides establish to be as likely to give a toy to an ain-race when compared to an other-race member, and information technology was not until five years of age that children displayed an overt bias (i.e., children were more prosocial towards an ain-race member as compared to an other-race member)16.
I way to further understand how infants respond to pupillary signals is to measure the brain processes involved in infants' detection of pupillary changes in others. Thus, one goal of the current study was to examine the neural correlates of processing pupillary changes in others by measuring localized cortical brain responses using functional about-infrared spectroscopy (fNIRS). Prior piece of work with adults using functional magnetic resonance imaging (fMRI) shows that especially the correct superior temporal sulcus, a region implicated in processing a broad array of dynamic social information from faces, including emotional and gaze cues17, besides responds to changes in student size18,xix. In this context, information technology is important to emphasize that, similar to adults, there is evidence from previous studies with infants showing that the superior temporal cortex, especially in the right hemisphere, is involved in middle gaze and emotion processing from early in infancy20,21. On the basis of the abovementioned studies examining the neural correlates of processing pupillary changes in adults and given the neural sensitivity to dynamic eye gaze and emotional cues in the superior temporal cortex previously demonstrated in infantstwenty,22,23, nosotros hypothesized that observed changes in pupil size volition consequence in differential brain responses in infants' superior temporal cortex (STC). Furthermore, considering the lateralization of STC responses reported in infants and adults20,21,24, we decided to examination for encephalon response lateralization past including hemisphere (left and right) equally a factor in our fNIRS analyses.
With respect to the brain procedure involved when encountering faces of other-race individuals, our utilise of fNIRS equally the neuroimaging method with infants limits our investigation to cortical brain regions and does not permit u.s.a. to paradigm responses from subcortical brain structures such as the amygdala25. I cortical region that is commonly engaged during interracial interactions in adults is the dorsolateral prefrontal cortex (dlPFC)26. This region is by and large implicated in cognitive control and its enhanced action during interracial contact is thought to reflect both cocky-regulatory processes, involved in attenuating racial bias during interracial encounters26,27, and increased effort, due to lack of familiarity and exposure to other-race individuals28. Although the prefrontal cortex is known to show protracted structural and functional development extending well beyond infancy, testify is mounting that prefrontal regions are functionally agile from earlier than previously thought29, and may specifically be involved in cerebral processes reflecting novelty detection during infancy30. We therefore hypothesized that 9-month-old infants prove enhanced responses in dlPFC when processing other-race stimuli.
Taken together, the electric current study was designed to shed light on the nature and early on development of sensitivity to pupillary cues by examining the brain processes involved in and the influence of racial context on infants' responses to observed pupillary changes in social partners. In club to achieve this, we adapted an experimental paradigm previously used with adults11,31. We presented 9-month-sometime infants with own-race and other-race eyes that were either dilating or constricting, while measuring their brain responses in frontal and temporal cortices using fNIRS. Specifically, we used Japanese, Asian, eyes as the other-race comparison. Asian optics were selected as the experimental out-group because of previous adult work that has used this comparison and shown differences in behavioral response to own- versus other-race stimulieleven. In addition, previous research with infants has shown that past nine months of age, White infants with express other-race exposure show a reduced power to identify individuals that are Asian14. Finally, Asian eyes stimuli used in the current study are controlled for a number of perceptual confounds (eye whites, iris, and pupil size), to further ensure that infants are responding to relatively subtle racial differences and not to coarse perceptual differences when processing own-race and other-race eyes.
Based on previous research that has shown that in that location is an enhanced activation in the STC during student mimicry in adults18,24 and work that has shown that behaviorally, infants merely respond to educatee dilation (and not pupil constriction)vii, we hypothesized that infants would evidence enhanced STC responses to pupil dilation24. Moreover, given the behavioral findings that pupil dilation mimicry is greatest for own race faces11,31, we hypothesized that STC activation to pupil dilation may be express to, or at least enhanced when, viewing own-race eyes dilate.
Finally, we also examined whether, similar to adults, infants recruit frontal brain regions linked to cerebral control (dlPFC) when processing other-race eyes. Examining this in infants tin can inform the question as to whether such an effect on cerebral control exists in the human encephalon well before children display an overt social preference for own-race members16. If this were the case then this would indicate that dlPFC recruitment is the result of beingness unfamiliar with other-race faces rather than having overt racial (dis)preferences/biases27,28,32.
Results
fNIRS Analysis
To analyze our fNIRS information nosotros conducted an jitney repeated measures ANOVA with encephalon region (STC, dlPFC), hemisphere (left, right), race (own, other), and pupil (constriction, dilation) as within-subject factors. This assay revealed a four-way interaction betwixt brain region, hemisphere, race, and pupil, F(1, 26) = 4.93, p = 0.035, ηii = 0.sixteen. To follow up on this interaction, which suggests that the educatee and race factors of involvement interact with brain region, we carried out repeated measures ANOVAs for the two brain regions separately.
STC
For the STC region, we conducted a repeated measures ANOVA with hemisphere (left, right), race (own, other), and pupil (constriction, dilation) as within-subject field factors. This analysis did non reveal any significant effects (all p-values > 0.077). Although we failed to observe an event in this analysis, we yet decided to acquit split repeated measures ANOVAs for ain-race and other-race optics in order to test our hypothesis that infants' sensitivity to pupillary changes in others is affected by race and might be more pronounced for own-race eyes. In this analysis, nosotros obtained a meaning interaction between hemisphere and pupil only in the own-race context, F(ane, 26) = iv.43, p = 0.045, ηtwo = 0.15. Specifically, own-race educatee dilation evoked greater responses than own-race constriction in the right hemisphere (ain-race dilation: K = ii.12 μM, SE = 1.99, own-race constriction: M = −0.68 μM, SE = 0.96), whereas the opposite blueprint was seen in the left hemisphere, where own-race constriction evoked greater responses than own-race dilation (own-race dilation: M = −1.84 μM, SE = 0.97, own-race constriction: 1000 = 1.84 μM, SE = i.86; see Fig. 1). For the other-race context, there were no pregnant effects (all p-values > 0.19).
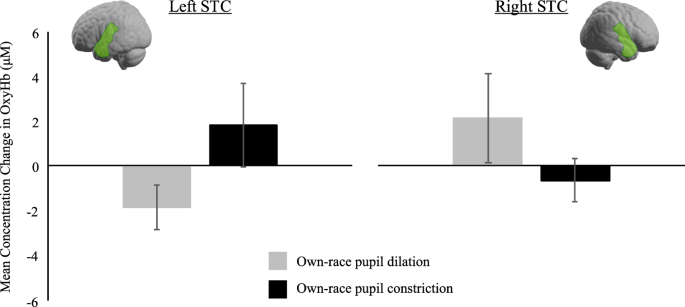
This shows the mean concentration changes in oxy-Hb in the left and right STC in response to ain-race pupil dilation and constriction and the judge cortical location. Note, error bars signal standard errors.
We also assessed whether STC responses were impacted by other-race experience. For this purpose, we conducted an boosted repeated-measures ANOVA with STC responses to own-race pupillary changes (dilating, constriction) and hemisphere (left, right) equally a within-subjects factors and other-race experience (at least once a calendar week, less than three times a month) as a between-subjects factor. This analysis revealed that other-race feel did not significantly bear on STC responses (p-values > 0.22).
dlPFC
For the dlPFC region, we conducted a repeated measures ANOVA with hemisphere (left, correct), race (own, other), and pupil (constriction, dilation) equally within-subject factors. Our analysis revealed a main effect of race on brain responses in the dlPFC region, F(one, 26) = 6.26, p = 0.019, η2 = 0.19, with other-race centre stimuli evoking greater responses (M = 2.48 μM, SE = 0.86) than own-race eye stimuli (Chiliad = 0.44, μM, SE = 0.69; see Fig. two). There were no other main effects or interactions (p > 0.068)
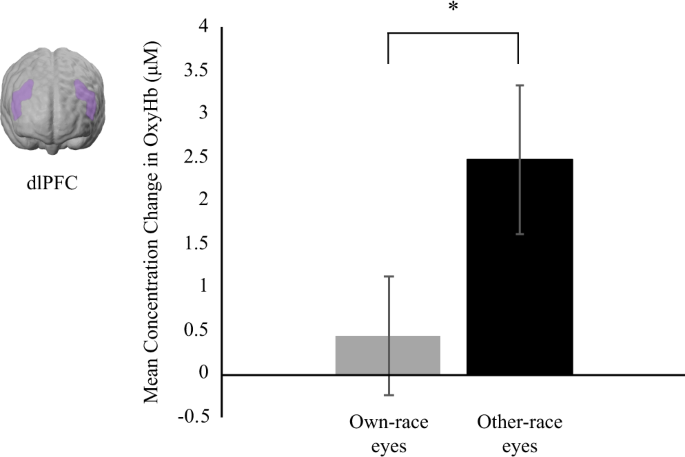
This shows the mean concentration changes in oxy-Hb in the dlPFC in response to own-race and other-race eyes and the approximate cortical location. Note, *indicates p-value < 0.05 and error confined signal standard errors.
Like to as we reported above for the STC analyses, we likewise assessed whether differential brain responses in the dlPFC were affected past other-race feel. To exercise this, we conducted an additional repeated measures ANOVA with dlPFC response to race (own, other) equally a within-subjects factor and other-race experience (at least once a week, less than three times a month) every bit a between-subjects factor. This analysis showed that other-race experience did not significantly bear on dlPFC responses to own- and other-race eyes (all p-values > 0.20).
Temporal Parietal Cortex
As an additional analysis of the fNIRS data, a repeated measures ANOVA with hemisphere (left, right), race (ain, other), and pupil (constriction, dilation) as within-subject factors was conducted in an additional encephalon region, the temporal parietal cortex (TPC). This brain region was selected because prior piece of work has identified that the TPC is sensitive to information regarding mental states33,34. However, this analysis did not reveal whatever significant effects (all p-values > 0.16).
Furthermore, in each of the three regions of involvement (dlPFC, STC and TPC) we assessed effects on deoxy-Hb concentration changes across experimental conditions. This analysis revealed no pregnant furnishings for the deoxy-Hb concentration changes (all p-values > 0.10; see supplemental materials for further information). The absence of deoxy-Hb effects is in agreement with a number of infant fNIRS studies that also did not notice condition furnishings on deoxy-Hb35.
Looking Time Analyses
In addition to our primary analysis of the fNIRS information we carried out an analysis of looking fourth dimension (duration of attention to the middle stimuli) coded from video. Specifically, we conducted a repeated measures ANOVA with race (own-race eyes, other-race eyes) and pupillary change (dilating, constricting) as within-subjects factors, to assess whether infants displayed whatever systematic differences in looking time (duration of attention/fixation on eye stimuli). This analysis revealed a significant interaction between the factors race and pupillary change, F(ane, 26) = ix.64, p = 0.005, ηii = 0.27. At that place were no master effects of race or pupillary modify (p-values > 0.48). A follow-up analysis performed by using paired-samples t-tests separately for ain- and other-race optics revealed that for own-race eyes, infants looked significantly longer at student dilation (M = v.99 seconds, SE = 0.07) than at educatee constriction (G = 5.69 seconds, SE = 0.13), t(26) = −ii.40, p = 0.024. In contrast, for other-race eyes, infants looked significantly longer to pupil constriction (Chiliad = 5.91 seconds, SE = 0.11) than to educatee dilation (Grand = v.72 seconds, SE = 0.12), t(26) = 2.32, p = 0.028 (see Fig. 3).
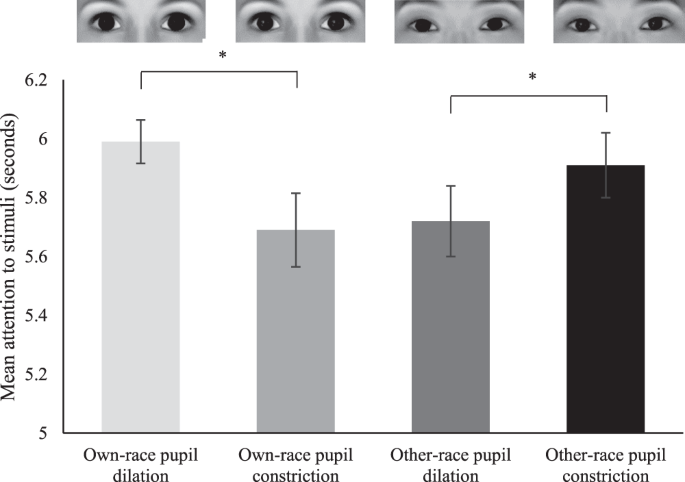
This shows the mean looking time to pupillary changes (dilation and constriction) for both own-race and other-race eyes. Please note that infants viewed photographic images of real optics (meet Methods) and that the eye images shown here were computer generated with the FaceGen software (https://facegen.com) for illustrative purposes. Notation, *indicates p-values < 0.05 and error bars betoken standard errors.
Considering the obtained looking time differences reported to a higher place, we carried out an boosted correlation analysis to examine potential associations between looking fourth dimension and brain responses measured by fNIRS. In detail, given our a priori hypothesis about the right STC and the pattern of the observed response that suggests that the correct STC response was enhanced to ain-race pupil dilation when compared to own-race constriction, we conducted a Pearson'southward correlation between correct STC to ain-race dilating eyes and looking time to own-race dilating eyes to examination if the STC response observed was impacted past attentional processes. However, this analysis showed that looking time and correct STC response to own-race dilating eyes were not significantly correlated (r(26) = 0.09, p = 0.66). This indicates that the looking fourth dimension increment is unlikely to account for the enhanced brain response seen to dilating own-race eyes.
Discussion
The electric current study investigated the neural basis of processing others' pupillary changes and how race impacts these processes in infancy. We measured 9-calendar month-one-time infants' brain responses to dilating and constricting pupils in the context of viewing own-race and other-race eyes. Our fNIRS results show that merely when responding to own-race eyes, infants' brains distinguished between dilating and constricting pupils. Specifically, infants showed enhanced responses in the correct superior temporal cortex when observing ain-race educatee dilation and in the left superior temporal cortex when observing own-race pupil constriction. Our finding of a pupil change-sensitive brain response localized to superior temporal brain regions in infants is in line with previous work with adultsxviii,19,36,37, suggesting the early ontogenetic emergence of brain function related to processing pupillary change cues. Furthermore, our results add to a growing body of enquiry with infants, demonstrating that superior temporal brain regions are critically involved in processing social information from faces including centre gaze cues20,22.
Information technology is important to emphasize that our baby data are the offset to show that superior temporal cortex responses to others' pupillary changes are express to own-race stimuli, because previous fMRI research with adults but examined encephalon responses in an own-race contexteighteen,xix. Nonetheless, this finding is in line with previous fMRI work with adults, demonstrating that the amygdala is merely sensitive to pupillary signals in the context of ain-species faces but not in the context of other-species (cat) faces37. Moreover, the current finding with infants is in agreement with behavioral piece of work with adults, showing that pupil mimicry as well equally trust decisions are modulated by race11,31. The obtained absence of a discriminatory brain response in the other-race context concurs with previous research with infants of similar ages, showing that face identity and emotion discrimination is dumb when other-race stimuli are employed12,13,14. It is thus possible that the current results, in conjunction with previous piece of work, index a more general impairment of infants' social perceptual processes in the context of other-race faces.
Still, it should be mentioned that our assay of infants' looking time responses indicates that infants are able to discriminate between pupil dilation and constriction regardless of racial context, because there are meaning differences in looking time betwixt dilation and constriction for both other-race and own-race eyes. However, while our looking time results bear witness that discrimination of pupillary change occurs in both racial contexts, the observed effects on looking time depend on racial context. Specifically, for own-race optics infants displayed an increase in looking time to student dilation, whereas for other-race eyes infants showed an increase in looking time to pupil constriction. This pattern of looking time effects is interesting in the low-cal of research showing that large pupils tend to exist perceived equally more than positive, whereas small pupils tend to be perceived every bit more negative or fifty-fifty angry4,six,nineteen,36,37,38. Information technology may thus be possible that larger pupils receive heightened attention from infants when seen during an interaction with an own-race (in-group) member because information technology is a potentially positive signal, whereas smaller pupils receive heightened attention during an interaction with an other-race member considering it is a potentially negative or threatening signal. However, information technology should be noted that previous work has not found differences in looking time to optics when viewing partners with dilating as compared to constricting pupils; therefore, this rather speculative proposal should be thoroughly investigated in time to come piece of work past manipulating the facial expressions of the person displaying changes in student size39. In any case, the assay of the looking fourth dimension data, which was non the primary focus of the current fNIRS study, provides behavioral evidence that race impacts the processing of pupillary changes in infants.
Though these results using looking time provide additional behavioral insights into how race impacts the processing of pupillary changes, i vital question remains. Namely, does infants' superior temporal cortex response when viewing pupillary changes, especially during dilation, correlate with infants' own pupillary response in the sense of pupil dilation mimicry every bit seen in prior babe work using pupillometry? Because that we did not concurrently measure infants' pupillary changes when viewing the middle stimuli in our study, we could not directly assess pupil mimicry and its neural correlate in our infant sample. However, there is fMRI work with adults showing that STC action correlates with one'south own pupillary changes18, suggesting that such a link may be. Information technology is therefore possible that infants' observed enhanced response in STC when viewing ain-race pupil dilation may reverberate brain processes linked to student mimicry. In this context, it is likewise important to mention that enhanced brain response seen in the correct STC did not correlated with the heightened looking time observed in response to own-race pupil dilation. Thus, though it is possible that the STC response is associated with pupillary changes in the infant observer, the STC response is non linked to looking time.
Our results further evidence that infants recruit the dorsolateral prefrontal cortex, a brain region linked to cognitive control functions, when processing other-race pupillary changes. This is in line with previous fMRI enquiry with adults and may suggest that interracial interactions afford greater cognitive control or try from the infants26. This finding may inform a debate in the developed literature regarding the nature of dlPFC interest during interracial contact, which could either be construed as the effect of being unfamiliar with other-race faces or from the development of a racial bias or stereotype that requires cerebral command26,27,28. The current testify for dlPFC involvement during interracial interactions in infants appears to favor the unfamiliarity (or novelty) view because, as outlined in the introduction, infants at this age do not display whatever overt behavioral preferences for own-race members or (dis-)preferences for other-race members; and, it is unlikely that infants take acquired developed-like racial stereotypes they need to suppress through exerting cerebral control during interracial contact40. Previous fNIRS inquiry with infants shows that presenting novel stimuli results in enhanced dlPFC responses30, tentatively supporting the interpretation that enhanced dlPFC responses are related to infants being unfamiliar and probable lacking contiguous interaction experience with individuals from the other-race. Nevertheless, it is possible that the recruitment of dlPFC during interracial interactions is initially a result of existence unfamiliar with some other-race individual, merely later develops into reflecting the suppression of racial stereotypes. Clearly, more than inquiry across a wider historic period range is required to test the development of dlPFC function during interracial interactions.
At this point it is important to mention that experience with other-race individuals as measured through parental report did not take an effect on infants' brain responses in the current study. Previous inquiry has reported that infants growing up in the urban United States spend on boilerplate 92% of their face-to-face interactions with own-race individuals (encounter Rennels & Davis, 2008). It is thus possible that even though 55.5% of parents in the current written report reported that their infants had some exposure to other-race individuals these interactions may not have been long or engaging enough to facilitate infants' processing of information from other-race faces.
One promising arroyo to further test the nature of dlPFC involvement and cognitive control during interracial interactions, would be to deport out grooming studies with infants. In fact, novel grooming studies, such as exposing infants to books that identify individuals from other-races, are effective strategies to better the recognition of other-race individualsxl. It will be important to examine whether this kind of training transfers to the electric current context and whether reduced dlPFC recruitment may be seen after the training. Moreover, with respect to the role of experience, it volition be disquisitional to extend the current research to Asian (Japanese) infants in order to examine whether the effects seen in the electric current study generalize beyond race. Previous research using the same stimuli has found similar patterns of perceptual narrowing across Asian and White infants; such that, White and Asian infants were able to recognize facial identities of other-race faces at 6 months of age (eastward.thousand., Asian infants were able to recognize both White and Asian faces) but by ix months of historic period both sets of infants were just able to identify own-race faces14,41. Given the previous work, it would be interesting to examination if Asian infants show the same blueprint of results (e.grand., recruit dlPFC in response to White optics and are able to distinguish pupillary cues only in the context of Asian eyes).
In decision, the current study identifies the superior temporal cortex as a brain region involved in infants' processing of pupillary changes from others. Our data further demonstrate that, early in evolution, brain processes of responding to pupillary changes in others are impacted by race and that inter-racial eye-based interactions may beget greater cognitive command or effort from infants, perhaps due to their novelty. This critically informs our understanding of the neurodevelopmental origins of pupil mimicry and highlights the impact that grouping-membership cues such as race accept on processing social information from the eyes.
Method
20-seven infants (14 girls, thirteen boys; 1000 [age] = 9 months, 23 days; ranging from nine months, 3 days to ten months, 21 days) were included in the final sample used for analysis. All of the participants were White. For the present study, we administered a parental report measure of other-race exposure to appraise infants experiences with other-race individuals and the majority of infants had minimal feel with whatsoever other-race individuals (55.half-dozen% interacted with other-race individuals more than once per calendar week; 44.4% interacted with other-race individuals less than 1–3 times per month). All participants were born at term, with normal nascence weight (>2,500 g), and did non have whatsoever hearing or visual impairments. Nineteen additional infants were tested just were excluded from the nowadays analyses. Note that this attrition rate is similar to previous infant fNIRS studies employing three or more experimental conditions42,43,44. Out of the 19 excluded infants, virtually were excluded because they failed to reach our pre-determined looking benchmark of attention to the visual presentation on the screen for a minimum of 60% of the trial (4.two s.) for at least ii trials per condition (north = xiii) and the others were excluded for being fussy or upset throughout the experiment (n = 6). Participants were recruited from a large database of infants and children in a mid-sized college town in the mid-Atlantic region of the US. All parents gave informed consent for their infants to participate in accordance with the Declaration of Helsinki and infants received a small toy for their participation. All procedures were approved by and carried out in accordance with The University of Virginia Institutional Review Board for Social and Behavioral Sciences.
Stimuli
Stimuli were adapted from previous behavioral studies of student mimicry in adults11,31. These stimuli consisted of images of the eye regions cropped from full-face neutral expression White (own-race) and East Asian (other-race) actors, onto which dynamically changing pupils were superimposed (please refer to the following papers for example stimuli: Kret, Fischer & De Dreu, 2015; Kret & De Dreu, 2017). Annotation that a series of controls were put in place by Kret and colleagues in social club to reduce possible perceptual confounds: (1) both own-race and other-race optics were identical for the inner features of the optics (eye white, iris, and pupil), (2) the stimuli were presented equally gray-scale in order to reduce perceptual differences due to contrasting skin tone and eye color, and (3) although there were private differences across actors for center shape, previous analyses bear witness that in that location is no statistical difference in the amount of eye white visible across own-race and other-race eyes11.
Each pupillary stimulus presentation consisted of three stages: (1) starting with a static neutral sized pupil (five mm) presented for 3 seconds, (ii) followed by a dynamic pupil size modify, which could either exist a dilation (going from 5 to 7 mm) or a constriction (going from 5 to 3 mm), presented over the course of 1.5 seconds, (3) followed past a static pupil, in either the fully dilated (7 mm) or the constricted (3 mm) state, presented over 2.5 seconds (run into Fig. 4 for an overview of the experimental paradigm). Please note, the timing for the experimental stimulus presentation is longer in this experiment compared to previous eye-tracking experiments that accept used the aforementioned stimuli11,24,31. This adjustment was made in social club to business relationship for the delay in the hemodynamic response measured by fNIRS. Pupil sizes were selected to reflect the normal range of pupillary fluctuationxi,31. Five different inanimate objects (vegetables) were used as the inter-stimulus interval images. These images have been used in previously published fNIRS studies examining facial processing in infancy23,45,46 and have served every bit an established non-social inter-stimulus interval. Note that while a non-social inter-stimulus interval was employed and any changes in infants' brain responses reflect a change from the not-social stimulation, in line with previous studies, we did not employ a baseline subtraction method whereby the concentration changes measured during the non-social inter-stimulus interval were subtracted from the four different heart stimulus conditions. A shaking rattle video clip (from Tobii software, Sweden) and accompanying audio (three tones ranging from 109 Hz to 262 Hz) was presented subsequently four experimental trials. This was used to regain infants' attending and orient them to the middle of the screen. The videos were 500 pixels wide and 300 pixels high, resulting in an eye region which is 13.v cm wide and 6 cm high on the estimator screen. Please note, the sizes of the stimuli and pupillary changes were selected based on prior work that has been successful in eliciting differential behavioral responses in educatee mimicry for similar historic period infants7.
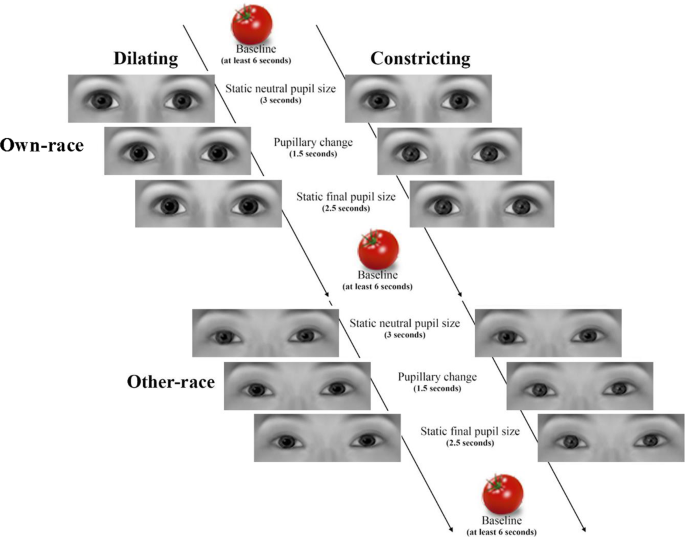
Illustration of the experimental process used in the current study. Please note that infants viewed photographic images of real eyes (encounter Methods) and that the eye images shown hither were computer generated with the FaceGen software (https://facegen.com) for illustrative purposes.
Procedure
Infants sat on their parents' laps in a quiet, dimly lit room, at a distance of approximately lx cm from the screen (23-inch monitor). The distance of the babe from the screen and size of the eye stimuli were selected to reflect the experience of a close-range face-to-confront interactionxi. A small plastic teething ring was available for each infant to concord during the study to increase attentiveness to the stimuli and decrease bodily movements during the experiment47,48.
The experimental epitome was presented by using the Presentation software bundle (Neurobehavioral Systems, United states). Infants were presented with a total of 32 heart stimuli (8 ain-race/constricting, 8 other-race/constricting, viii own-race/dilating, 8 other-race/dilating). Each experimental trial lasted seven seconds. Between each experimental trial, there was a brief bell sound (virtually 150 milliseconds and 600 Hz) and a dynamic non-social inter-stimulus interval (which was jittered and presented for at least six seconds)23,45,46,49. The sequence of the presentation was pseudo-randomized so that no more than 2 consecutive trials were from the same type of race (own or other) or pupillary change (dilation or constriction). To ensure that the infants looked at the screen, each experimental trial was started manually past the experimenter when the infant attended to the screen, resulting in variable inter-stimulus intervals. The entire experimental session took approximately 10 minutes.
Information acquisition
Infants' fNIRS information were recorded using a NIRx Nirscout system and NirStar conquering software. The fNIRS method quantifies concentration changes of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) in the cerebral cortex. This is done by utilizing specific frequencies of near-infrared low-cal that are selectively absorbed past oxy-Hb and deoxy-Hb (for an overview meet50). The NIRx Nirscout arrangement used contains 16 source-detector pairs, arranged to effect in a total of 49 channels (see Supplemental Materials). A fabric cap (Easycap) configured the source-detector pairs approximately 2.five centimeters apart in the frontal and temporal cortices in both hemispheres. Data were recorded at a sampling rate of 3.91 Hz. Almost-infrared lite-emitting diodes were emitted at 2 wavelengths at 780 nm and 830 nm with a power of 20 mW/wavelength. The fNIRS arrangement automatically fabricated adjustments for low-cal intensity in guild to provide optimal gain. A camera mounted above the screen recorded infants' beliefs during the experiment and allowed for later coding of attention throughout the experiment.
Data analysis
Infants' attention during the fNIRS paradigm was coded offline past a trained research assistant from video recordings of the experimental session. Specifically, the fourth dimension the babe spent looking at each experimental trial was recorded. To appraise the reliability of the attentional coding washed by the master coder, an boosted trained coder also coded babe looking time from a randomly selected subsample of infants (25.9%; n = 7). This analysis showed that inter-rater reliability was splendid (Cronbach's α = 0.94). Note, trials were only included if the infant looked for more than than 60% of the presentation time of an individual experimental trial (4.2 seconds). For each infant, the boilerplate looking time across all included trials for each experimental condition was calculated and used every bit a measure of attention to stimuli in the Results department.
In improver, all trials were excluded that exhibited motion artifacts (determined past visual inspection of the hemodynamic response across all individual channels). In gild for infants to be included in our data analysis, they had to contribute at least two artifact free trials with acceptable attentiveness per status (ain-race/dilating, own-race/constricting, other-race/dilating, other-race/constricting). This threshold of two artifact costless trials for which infants looked for at least 60% of the time is similar to previous fNIRS research with infants51 and all trials that did non reach this threshold were removed prior to data analysis. The final sample included 27 infants that on boilerplate contributed data for a total of 17.nineteen trials, SD = 6.77 (M trials per status = 4.30, SD = one.69). Note, we conducted a repeated measures ANOVA to assess if there were whatsoever systematic differences in attention to trials across conditions and nosotros found no significant differences in the number of trials included across conditions (p = 0.91).
The fNIRS data were analyzed using the Matlab-based software nilab2 (see the following papers for other fNIRS data analyzed using this software)52,53. Data were filtered using a 0.2 Hz depression-pass filter (to remove fast fluctuations related to eye charge per unit) and a loftier-laissez passer filter of 0.07 Hz (to remove changes that were as well boring and related to migrate). Oxy-Hb and deoxy-Hb concentration changes were calculated for each condition using the modified Beer-Lambert law and baseline corrected (whereby all the experimental hemodynamic responses are set to begin at 0 mM with stimulus onset). We computed hemodynamic concentration changes in response to the stimulus conditions. The stimulus length was set up to 7 seconds (which reflects the stimulus duration from eyes onset to optics offset). The boxcar functions corresponding to the four stimulus conditions were convolved with a standard hemodynamic response function based on the stimulus length parameter54. In line with previous reports from vascular imaging in infants with the Bold contrast and optical topography55,56, we used parameters similar to those used in developed subjects, assuming a top response at v s (τ = i). The assay time window considered for computing concentration changes was set from 0 (stimulus onset) to 20 seconds (which takes into business relationship this lag in the hemodynamic response peak).
Analyses examining condition differences were conducted for both oxy-Hb and deoxy-Hb changes. Furthermore, regions of interest were created for the STC and the dlPFC in both hemispheres; these regions were based on previously published information regarding the cortical projection of the ten–xx EEG system57 and carried out by selecting the respective fNIRS channels (see Fig. 5). The channels selected for the STC approximately corresponded with the FT8 and C6 electrode positions in the correct hemisphere and FT7 and C5 electrode positions in the left hemisphere. The channels selected for the dlPFC approximately corresponded with the F4 and F6 electrode positions in the right hemisphere and the F3 and F5 electrode positions in the left hemisphere. Lastly, the channels selected for the control region, the TPC, approximately corresponded with the CP4 electrode position in the right hemisphere and the CP3 electrode position in the left hemisphere.
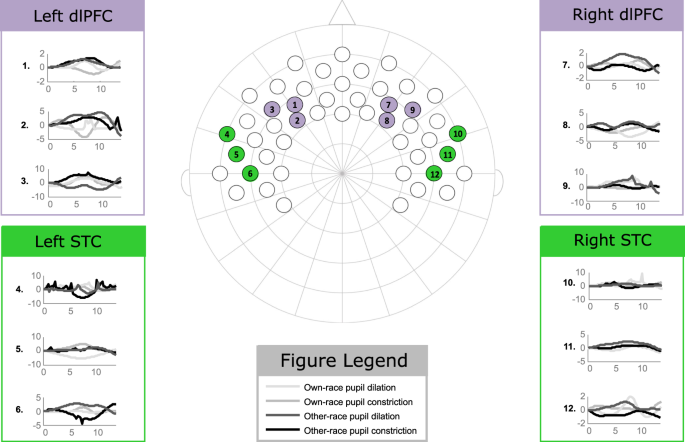
This shows the fNIRS channel placement with respect to the relevant 10–20 arrangement and the associated oxy-Hb hemodynamic response (the units for the y-centrality are in μM) across each experimental condition (the units for the x-centrality are seconds). The ROIs are color-coded as follows: dlPFC (purple), STC (green).
Information Availability
For more than data on study stimuli please contact MK. The dataset for the present study is available from the corresponding author (TG) at request.
References
-
Farroni, T., Csibra, One thousand., Simion, F. & Johnson, G. H. Eye contact detection in humans from nativity. Proceedings of the National University of Sciences 99, 9602–9605, https://doi.org/10.1073/pnas.152159999 (2002).
-
Farroni, T. et al. Babe cortex responds to other humans from soon afterward birth. Scientific Reports 3, https://doi.org/x.1038/srep02851 (2013).
-
Grossmann, T. The eyes every bit windows into other minds: An integrative perspective. Perspectives on. Psychological Scientific discipline. 12, 107–121, https://doi.org/ten.1177/1745691616654457 (2017).
-
Hess, E. H. The role of student size in communication. Scientific American 233, 110–119 (1975).
-
Kahneman, D. & Beatty, J. Student diameter and load on retentivity. Science 154, 1583–1585, https://doi.org/x.1126/science.154.3756.1583 (1966).
-
Kret, Thou. E. The function of pupil size in advice. Is there room for learning? Noesis and Emotion, 1–vii, https://doi.org/10.1080/02699931.2017.1370417 (2017).
-
Fawcett, C., Arslan, M., Falck-Ytter, T., Roeyers, H. & Gredebäck, G. Human eyes with dilated pupils induce pupillary contagion in infants. Scientific Reports seven, https://doi.org/ten.1038/s41598-017-08223-3 (2017).
-
Fawcett, C., Wesevich, V. & Gredebäck, Grand. Pupillary contagion in infancy: Evidence for spontaneous transfer of arousal. Psychological Science 27, 997–1003, https://doi.org/10.1177/0956797616643924 (2016).
-
Bradley, M. M., Miccoli, L., Escrig, M. A. & Lang, P. J. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–607, https://doi.org/10.1111/j.1469-8986.2008.00654.10 (2008).
-
Kang, O. & Wheatley, T. Student dilation patterns spontaneously synchronize across individuals during shared attention. Journal of Experimental Psychology: Full general 146, 569, https://doi.org/x.1037/xge0000271 (2017).
-
Kret, M. E., Fischer, A. & De Dreu, C. Pupil mimicry correlates with trust in in-group partners with dilating pupils. Psychological Science 26, 1401–1410, https://doi.org/x.1177/0956797615588306 (2015).
-
Kelly, D. J. et al. Three‐month‐olds, but non newborns, prefer own‐race faces. Developmental Science viii, 31–36, https://doi.org/ten.1111/j.1467-7687.2005.0434a.10 (2005).
-
Vogel, 1000., Monesson, A. & Scott, L. S. Edifice biases in infancy: The influence of race on face and vox emotion matching. Developmental Science xv, 359–372, https://doi.org/10.1111/j.1467-7687.2012.01138.x (2012).
-
Kelly, D. J. et al. The other-race effect develops during infancy evidence of perceptual narrowing. Psychological Scientific discipline 18, 1084–1089, https://doi.org/10.1111/j.1467-9280.2007.02029.10 (2007).
-
Liu, S. et al. Similarity and divergence in the processing of aforementioned-and other-race faces as revealed past heart tracking in 4-to 9-calendar month-olds. Journal of Experimental Child Psychology 108, 180–189, https://doi.org/10.1016/j.jecp.2010.06.008 (2011).
-
Kinzler, K. D. & Spelke, E. S. Do infants show social preferences for people differing in race? Cognition 119, 1–ix, https://doi.org/10.1016/j.cognition.2010.x.019 (2011).
-
Allison, T., Puce, A. & McCarthy, G. Social perception from visual cues: function of the STS region. Trends in Cognitive Sciences iv, 267–278, https://doi.org/x.1016/S1364-6613(00)01501-1 (2000).
-
Harrison, N. A., Gray, M. A. & Critchley, H. D. Dynamic pupillary exchange engages brain regions encoding social salience. Social Neuroscience four, 233–243, https://doi.org/10.1080/17470910802553508 (2009).
-
Harrison, N. A., Wilson, C. E. & Critchley, H. D. Processing of observed student size modulates perception of sadness and predicts empathy. Emotion 7, 724, https://doi.org/10.1037/1528-3542.7.4.724 (2007).
-
Grossmann, T. et al. Early on cortical specialization for contiguous advice in human infants. Proceedings of the Majestic Social club B: Biological Sciences 275, 2803–2811, https://doi.org/ten.1098/rspb.2008.0986 (2008).
-
Carlsson, J., Lagercrantz, H., Olson, Fifty., Printz, G. & Bartocci, M. Activation of the right fronto-temporal cortex during maternal facial recognition in young infants. Acta Paediatrica (Oslo, Norway: 1992 ) 97, 1221–1225, https://doi.org/x.1111/j.1651-2227.2008.00886.x (2008).
-
Grossmann, T., Lloyd-Pull a fast one on, S. & Johnson, 1000. H. Brain responses reveal young infants' sensitivity to when a social partner follows their gaze. Developmental Cerebral Neuroscience 6, 155–161, https://doi.org/10.1016/j.dcn.2013.09.004 (2013).
-
Nakato, Eastward., Otsuka, Y., Kanazawa, S., Yamaguchi, M. K. & Kakigi, R. Singled-out differences in the pattern of hemodynamic response to happy and aroused facial expressions in infants—A nigh-infrared spectroscopic written report. NeuroImage 54, 1600–1606, https://doi.org/ten.1016/j.neuroimage.2010.09.021 (2011).
-
Prochazkova, E. et al. Educatee mimicry promotes trust through the theory-of-mind network. Proceedings of the National Academy of Sciences 115, E7265–E7274, https://doi.org/x.1073/pnas.1803916115 (2018).
-
Ferrari, M. & Quaresima, V. A brief review on the history of man functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage 63, 921–935, https://doi.org/10.1016/j.neuroimage.2012.03.049 (2012).
-
Kubota, J. T., Banaji, M. R. & Phelps, E. A. The neuroscience of race. Nature Neuroscience 15, 940–948, https://doi.org/10.1038/nn.3136 (2012).
-
Richeson, J. A. et al. An fMRI investigation of the affect of interracial contact on executive function. Nature Neuroscience half-dozen, 1323, https://doi.org/10.1038/nn1156 (2003).
-
Hugenberg, K., Young, S. K., Bernstein, M. J. & Sacco, D. F. The categorization-individuation model: an integrative account of the other-race recognition deficit. Psychological Review 117, 1168, https://doi.org/10.1037/a0020463 (2010).
-
Grossmann, T. The role of medial prefrontal cortex in early social knowledge. Frontiers in Homo Neuroscience 7, https://doi.org/10.3389/fnhum.2013.00340 (2013).
-
Nakano, T., Watanabe, H., Homae, F. & Taga, K. Prefrontal cortical interest in young infants' assay of novelty. Cerebral Cortex nineteen, 455–463, https://doi.org/x.1093/cercor/bhn096 (2009).
-
Kret, M. E. & De Dreu, C. Chiliad. Pupil-mimicry weather trust in partners: moderation by oxytocin and grouping membership. Proceedings of the Royal Society B 284, e20162554, https://doi.org/10.1098/rspb.2016.2554 (2017).
-
Naoi, N. et al. Cerebral responses to infant-directed voice communication and the upshot of talker familiarity. NeuroImage 59, 1735–1744, https://doi.org/10.1016/j.neuroimage.2011.07.093 (2012).
-
Saxe, R. & Kanwisher, N. People thinking most thinking people: the role of the temporo-parietal junction in "theory of listen". NeuroImage xix, 1835–1842 (2003).
-
Hyde, D. C., Simon, C. Eastward., Ting, F. & Nikolaeva, J. I. Functional Organization of the Temporal-Parietal Junction for Theory of Listen in Preverbal Infants: A About-Infrared Spectroscopy Study. Periodical of Neuroscience 38, 4264–4274, https://doi.org/10.1523/jneurosci.0264-17.2018 (2018).
-
Cristia, A. et al. An online database of infant functional near infrared spectroscopy studies: a community-augmented systematic review. Plos Ane eight, e58906, https://doi.org/x.1371/journal.pone.0058906 (2013).
-
Demos, Thousand. Eastward., Kelley, W. Grand., Ryan, S. L., Davis, F. C. & Whalen, P. Human amygdala sensitivity to the educatee size of others. Cerebral Cortex 18, 2729–2734, https://doi.org/10.1093/cercor/bhn034 (2008).
-
Amemiya, Southward. & Ohtomo, K. Outcome of the observed pupil size on the amygdala of the beholders. Social Cerebral and Affective Neuroscience 7, 332–341, https://doi.org/10.1093/scan/nsr013 (2012).
-
Harrison, N. A., Singer, T., Rotshtein, P., Dolan, R. J. & Critchley, H. D. Pupillary contagion: central mechanisms engaged in sadness processing. Social Cerebral and Affective Neuroscience 1, v–17, https://doi.org/10.1093/scan/nsl006 (2006).
-
Prochazkova, East. et al. Reply to Mathot and Naber: Neuroimaging shows that educatee mimicry is a social phenomenon. Proceedings of the National University of Sciences 115, E11566–E11567, https://doi.org/x.1073/pnas.1815545115 (2018).
-
Heron-Delaney, M. et al. Perceptual preparation prevents the emergence of the other race effect during infancy. Plos 1 six, e19858, https://doi.org/ten.1371/journal.pone.0019858 (2011).
-
Kelly, D. J. et al. Development of the other-race event during infancy: Evidence toward universality? Journal of Experimental Child Psychology 104, 105–114, https://doi.org/10.1016/j.jecp.2009.01.006 (2009).
-
Southgate, V., Begus, K., Lloyd-Fox, S., di Gangi, V. & Hamilton, A. Goal representation in the infant brain. NeuroImage 85, 294–301, https://doi.org/x.1016/j.neuroimage.2013.08.043 (2014).
-
Lloyd-Play a trick on, S., Széplaki-Köllőd, B., Yin, J. & Csibra, Grand. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex 70, 35–48, https://doi.org/10.1016/j.cortex.2015.02.005 (2015).
-
Filippetti, 1000. L., Lloyd-Fox, Southward., Longo, 1000. R., Farroni, T. & Johnson, Thousand. H. Neural mechanisms of body awareness in infants. Cerebral Cortex 25, 3779–3787, https://doi.org/10.1093/cercor/bhu261 (2014).
-
Otsuka, Y. et al. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. NeuroImage 34, 399–406, https://doi.org/ten.1016/j.neuroimage.2006.08.013 (2007).
-
Nakato, East. et al. When exercise infants differentiate profile face from frontal face? A nigh‐infrared spectroscopic study. Human Brain Mapping thirty, 462–472, https://doi.org/x.1002/hbm.20516 (2009).
-
Altvater‐Mackensen, N. & Grossmann, T. Learning to match auditory and visual speech cues: Social influences on conquering of phonological categories. Child Development 86, 362–378, https://doi.org/10.1111/cdev.12320 (2015).
-
Krol, Thou. M., Monakhov, Yard., San Lai, P., Ebstein, R. P. & Grossmann, T. Genetic variation in CD38 and breastfeeding experience interact to impact infants' attending to social centre cues. Proceedings of the National Academy of Sciences 112, E5434–E5442, https://doi.org/10.1073/pnas.1506352112 (2015).
-
Kobayashi, One thousand., Otsuka, Y., Kanazawa, Due south., Yamaguchi, Thou. K. & Kakigi, R. Size-invariant representation of face in baby brain: an fNIRS-accommodation study. NeuroReport 23, 984–988, https://doi.org/10.1097/WNR.0b013e32835a4b86 (2012).
-
Lloyd-Fox, S., Blasi, A. & Elwell, C. Illuminating the developing brain: the past, present and time to come of functional well-nigh infrared spectroscopy. Neuroscience and Biobehavioral Reviews 34, 269–284, https://doi.org/10.1016/j.neubiorev.2009.07.008 (2010).
-
Ravicz, G. Thou., Perdue, K. L., Westerlund, A., Vanderwert, R. E. & Nelson, C. A. Infants' neural responses to facial emotion in the prefrontal cortex are correlated with temperament: a functional about-infrared spectroscopy study. Frontiers in Psychology 6, https://doi.org/ten.3389/fpsyg.2015.00922 (2015).
-
Grossmann, T., Oberecker, R., Koch, S. P. & Friederici, A. D. The developmental origins of voice processing in the human brain. Neuron 65, 852–858, https://doi.org/x.1016/j.neuron.2010.03.001 (2010).
-
Altvater-Mackensen, Northward. & Grossmann, T. The office of left inferior frontal cortex during audiovisual speech communication perception in infants. NeuroImage 133, 14–20, https://doi.org/10.1016/j.neuroimage.2016.02.061 (2016).
-
Boynton, G. M., Engel, S. A., Glover, One thousand. H. & Heeger, D. J. Linear systems analysis of functional magnetic resonance imaging in human V1. Periodical of Neuroscience sixteen, 4207–4221 (1996).
-
Taga, Chiliad., Asakawa, One thousand., Hirasawa, Yard. & Konishi, Y. Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: A near-infrared optical topography report. Early on Human being Development 75, S203–210 (2003).
-
Homae, F., Watanabe, H., Nakano, T., Asakawa, K. & Taga, G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research 54, 276–280, https://doi.org/ten.1016/j.neures.2005.12.006 (2006).
-
Kabdebon, C. et al. Anatomical correlations of the international x–xx sensor placement system in infants. NeuroImage 99, 342–356, https://doi.org/10.1016/j.neuroimage.2014.05.046 (2014).
Author information
Affiliations
Contributions
C.Thou. and T.G. developed the report concept. All authors contributed to the study design. M.E.K. generously provided the study stimuli. C.Thou. acquired and analyzed the fNIRS data with supervision past T.G. and K.Chiliad. K.Thousand. and C.K. designed the figures. C.Yard. and T.G. drafted the manuscript, and One thousand.E.K. and K.K. provided critical revisions. All authors approved the last version of the manuscript for submission.
Respective author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional data
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed nether a Creative Commons Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatsoever medium or format, as long as you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and betoken if changes were made. The images or other tertiary party material in this article are included in the commodity'southward Creative Commons license, unless indicated otherwise in a credit line to the fabric. If material is not included in the article's Artistic Eatables license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you lot will demand to obtain permission straight from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/past/4.0/.
Reprints and Permissions
Well-nigh this article
Cite this article
Kelsey, C.M., Krol, K.M., Kret, G. et al. Infants' brain responses to pupillary changes in others are afflicted by race. Sci Rep ix, 4317 (2019). https://doi.org/ten.1038/s41598-019-40661-z
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-019-40661-z
Comments
Past submitting a comment yous agree to abide past our Terms and Community Guidelines. If yous find something calumniating or that does not comply with our terms or guidelines delight flag it as inappropriate.
hylandkintalind1961.blogspot.com
Source: https://www.nature.com/articles/s41598-019-40661-z
0 Response to "How Can I Tell if My Babies Eyes Are Dialited"
Post a Comment